If I define Thermodynamic beta in unit of second. Does this mean that:
1. Boltzmann constant k is unit-less?
2. T is in units of frequency (Hz) or Kelvin K?
In this case, is defining Thermodynamic beta β in unit of second a reason to make ℏ unitless? To make ℏ = 1 with no unit, which originally is 1.0545718×10−34m2kgs−1, should Boltzmann constant k be scaled appropriately?
This is the table of Student Friendly Quantum Field Theory and what I mentioned seems to belong to the hybrid case:
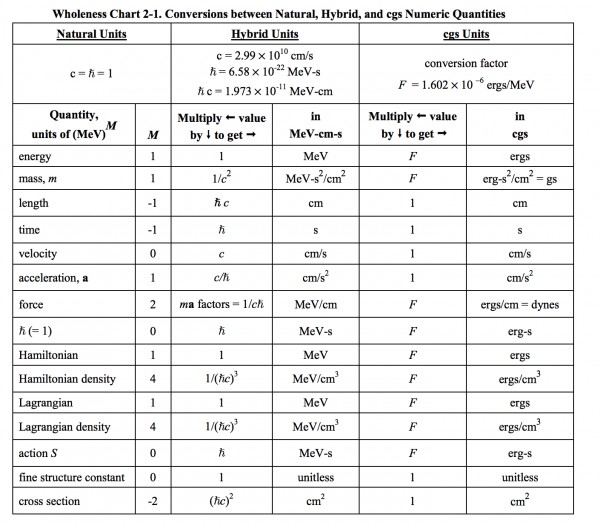
β=1/kT has the units of J−1, so ℏβ has the units of s. Redefining a scaled version of the Boltzmann constant by 1/k′=ℏ/k would give β′=1/k′T the units of s. However, when most of the time I would use ℏ independent of thermodynamic beta and vice versa. If I use ℏ=1, which is the cause of every scaling, setting k′=k/ℏ does work when ℏ and β is used together. But in classical statistical mechanics, no ℏ appears and β is used independently, why should β changed just because I want to set ℏ=1 unitless?
 Q&A (4908)
Q&A (4908) Reviews (205)
Reviews (205) Meta (440)
Meta (440) Q&A (4908)
Q&A (4908) Reviews (205)
Reviews (205) Meta (440)
Meta (440)